How Do Antibiotics Affect Gut Microbiome: Key Insights
How do antibiotics affect gut microbiome? This question has become increasingly relevant as we continue to learn more about the complex relationship between our health and the trillions of microorganisms living within us. In this blog post, we will explore the consequences of antibiotic use on gut bacteria and how antimicrobial stewardship interventions can help reduce resistance.
We will also explore antibiotic scarring and resistance genes, specifically examining Azithromycin’s delayed recovery time and increased compositional differences as well as Amoxicillin’s effects on patients with lower respiratory tract infections. Furthermore, we will discuss antimicrobial stewardship interventions that promote responsible prescribing practices among healthcare providers while encouraging collaborative efforts across multiple sectors to tackle antibiotic resistance.
Last but not least, this post will investigate probiotics as a potential solution for maintaining gut microbiome balance during antibiotic treatment. We’ll examine their role in preventing antibiotic-associated diarrhea and how they may help preserve our precious gut flora. Join us in exploring how do antibiotics affect gut microbiome and how to potentially mitigate their effects.
Table of Contents
- How do Antibiotics affect Gut Microbiome
- Disruption of Beneficial Microbial Communities by Antibiotics
- Overgrowth of Opportunistic Bacteria Such as Streptococcus and Lactobacillus Genera
- Antibiotic Scarring and Resistance Genes
- Antimicrobial Stewardship Interventions
- Probiotics – A Potential Solution
- Conclusion
How do Antibiotics affect Gut Microbiome
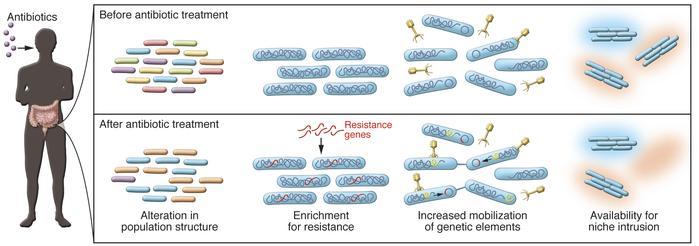
Antibiotics are commonly prescribed medications that target specific bacteria responsible for infections. However, their broad-spectrum nature also means they inadvertently affect other non-targeted bacterial species residing in our gut, leading to potential long-term consequences for overall well-being. Short courses of antibiotics can result in reduced diversity within the complex ecosystem of gut microbiota.
Disruption of Beneficial Microbial Communities by Antibiotics
One way how do antibiotics affect gut microbiome is it disrupts the balance between beneficial and harmful microbes living in our intestines. This disruption can lead to a decrease in beneficial microbial communities such as Bifidobacteria and Lactobacilli, which play essential roles in maintaining gut health and immune function. The loss of these helpful microbes may increase susceptibility to gastrointestinal issues like inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and even obesity.
Overgrowth of Opportunistic Bacteria Such as Streptococcus and Lactobacillus Genera
Another way how do antibiotics affect gut microbiome is by creating an environment where opportunistic pathogens thrive due to decreased competition from other microorganisms. For example, overuse or misuse of antibiotics has been linked with increased rates of Clostridioides difficile infection (CDI) – a severe form of diarrhea caused by the overgrowth of C.difficile bacteria in the gut. This bacterium can be hazardous, as it generates toxins that may harm the intestine’s lining, potentially causing dangerous inflammation and other complications.
Additionally, antibiotic treatment can result in an increase of bacteria resistant to antibiotics such as Streptococcus pneumoniae and Lactobacillus species, which may be difficult to eradicate with conventional treatments. These resistant bacteria are more difficult to treat with standard antibiotics, posing a significant threat to public health due to their potential spread within communities and healthcare settings.
It is critical for researchers to carry on investigating the intricate connections between medications and microbial populations in order to get a better comprehension of how do antibiotics affect gut microbiome and general well-being. In turn, this knowledge will help inform more targeted therapies that minimize harm while still effectively treating bacterial infections.
Antibiotic Scarring and Resistance Genes
One of the major concerns with “how do antibiotics affect gut microbiome”, is the long-term effect known as “antibiotic scarring”. A systematic review examining the impact of commonly prescribed antibiotics on gut microbiota found that azithromycin could delay recovery time and increase net compositional differences between pre and post-treatment samples.
This phenomenon highlights the importance of understanding how specific antibiotics may affect our delicate internal ecosystems.
Azithromycin’s Delayed Recovery Time and Increased Compositional Differences
Azithromycin, a widely prescribed antibiotic for respiratory infections, has been found to significantly alter gut bacterial composition and can take months after treatment to restore. In certain instances, the microbial changes induced by azithromycin may endure for several months following cessation of treatment.
The delayed recovery time associated with azithromycin use underscores the need for healthcare providers to carefully consider which antibiotics are most appropriate for their patients’ unique needs.
Amoxicillin’s Effects on Patients with Lower Respiratory Tract Infections
In addition to azithromycin, other common antibiotics such as amoxicillin have also been studied regarding their impacts on gut microbiome health. Research indicates that amoxicillin treatment in patients suffering from lower respiratory tract infections can lead to an increased abundance of certain bacterial species like Streptococcus pneumoniae while reducing overall microbial diversity within fecal samples. These findings emphasize that even short courses of antibiotic therapy can result in substantial shifts within our gastrointestinal flora.
Antibiotics can cause significant changes to the gut microbiome, including increased resistance genes and delayed recovery times. Moving on, antimicrobial stewardship interventions are necessary for promoting responsible prescribing practices among healthcare providers in order to tackle antibiotic resistance.
Antimicrobial Stewardship Interventions
In response to the growing public health concerns regarding antibiotic usage and its impact on our delicate internal ecosystems, countries like the UK have implemented antimicrobial stewardship interventions. These initiatives aim to promote responsible prescribing practices among healthcare providers while maintaining a balance within our gut microbiota.
By addressing this issue through collaborative efforts across multiple sectors such as agriculture, veterinary medicine, and environmental management industries alike, we can work together in tackling antibiotic resistance both domestically and internationally.
Promoting Responsible Prescribing Practices Among Healthcare Providers
Encouraging healthcare providers to use antibiotics judiciously and only when necessary is a key component of antimicrobial stewardship initiatives. This involves educating physicians about appropriate indications for antibiotic use, considering alternative treatments when possible, and ensuring that prescribed antibiotics are targeted toward specific pathogens rather than using broad-spectrum agents indiscriminately.
Additionally, these programs emphasize monitoring patient outcomes closely during treatment with antibiotics so that adjustments can be made if needed based on clinical response or potential side effects.
Collaborative Efforts Across Multiple Sectors To Tackle Antibiotic Resistance
The fight against antibiotic resistance requires cooperation from various stakeholders in different fields. For instance:
- Agriculture: Implementing policies that restrict the nontherapeutic use of antibiotics in livestock production helps reduce unnecessary exposure to these drugs within animal populations and their surrounding environments.
- Veterinary Medicine: Encouraging veterinarians to follow similar principles as human healthcare providers by promoting judicious prescribing practices ensures animals receive appropriate care without contributing to antibiotic resistance.
- Environmental Management: Developing strategies for proper disposal of antibiotics and other pharmaceuticals can minimize their presence in water sources, thus reducing the potential spread of resistant bacteria through environmental contamination.
By working together across these sectors, we can make significant strides toward preserving the effectiveness of antibiotics for future generations while maintaining a healthy balance within our gut microbiota. Probiotics have been found to play a role in maintaining gut microbiome balance and can help reduce the risk of antibiotic-associated diarrhea.
Probiotics – A Potential Solution
Research has demonstrated promising results regarding the potential benefits of probiotics, which are live microorganisms intended to support healthy digestive systems when consumed in adequate amounts. Studies have shown that probiotics may help mitigate the risk of antibiotic-associated diarrhea, which can range from mild discomfort to severe dehydration and electrolyte imbalances.
Probiotics as a Preventative Measure for Antibiotic-Associated Diarrhea
Several studies suggest that incorporating probiotics into one’s diet during and after antibiotic treatment can significantly reduce the risk of developing antibiotic-associated diarrhea. Probiotic strains such as Lactobacillus rhamnosus GG, Saccharomyces boulardii, and Bifidobacterium animalis subspecies lactis BB-12 have been found effective in reducing this risk by promoting gut bacteria balance.
- Lactobacillus rhamnosus GG: This strain is known for its ability to adhere to intestinal cells and produce antimicrobial substances that inhibit harmful bacteria growth.
- Saccharomyces boulardii: A yeast-based probiotic with anti-inflammatory properties that helps maintain gut barrier function while stimulating protective immune responses against pathogens.
- Bifidobacterium animalis subsp. lactis BB-12: This strain supports overall gastrointestinal health by enhancing natural defenses against harmful bacteria, improving digestion, and increasing resistance to infections.
The Role of Probiotic Supplementation in Maintaining Gut Microbiome Balance
Probiotics can be a key factor in sustaining the equilibrium of gut microorganisms during and following antibiotic therapy. By introducing beneficial bacteria into the gastrointestinal tract, probiotics help restore the disrupted microbial communities caused by antibiotics. This not only aids in preventing antibiotic-associated diarrhea but also supports overall gut health and immune function.
Research has shown that certain probiotic strains can reduce the duration of antibiotic-induced disruption to gut microbiota while promoting faster recovery of beneficial bacterial populations. Furthermore, some studies suggest that regular consumption of probiotics may even help decrease antibiotic resistance genes prevalence within fecal samples.
Incorporating probiotics into one’s daily routine through supplements or fermented foods like yogurt, kefir, sauerkraut, kimchi, and kombucha is an effective way to support a healthy gut microbiome amidst antibiotic treatments. Yet, before beginning any supplement routine, it is important to seek advice from a medical professional due to the fact that individual needs can be contingent on existing medications and health history.
Conclusion
To sum up, it is essential to be mindful of how do antibiotics affect gut microbiome and how they could lead to lasting repercussions. Minimizing any damage that could result from antibiotic use should be a priority, such as taking probiotics or consuming fermented foods. If you are concerned about the long-term impacts of antibiotics usage, speak with your doctor for further advice.